API and IP Newsletter
Contents
Recent P-IV filings
General information
Global Trends in R&D 2025
Cassava Sciences must face malicious prosecution lawsuit over Alzheimer's drug
Intellectual Property
T 2275/22 (Glaucoma II/SANTEN) 25-02-2025
Recent P-IV filings
On March 17, the FDA’s website published the list of new Paragraph IV (P-IV) filings. We track P-IV filings as they appear on the FDA site.
What are P-IV filings?
When a generic company submits an Abbreviated New Drug Application (ANDA), it must include a certification stating that one or more patents listed in the Orange Book for the brand-name drug are invalid, unenforceable, or not infringed by the generic product. This certification is referred to as a "Paragraph IV certification."
A Paragraph IV certification enables a generic drug manufacturer to challenge current patents listed in the Orange Book for brand-name drugs before they expire.
Our comments regarding recent P-IV filings are as follows.

General information
Global Trends in R&D 2025
This annual trend report from the IQVIA Institute assesses the trends in R&D funding, clinical trial activity, and new drug approvals and launches. It also examines the efficiency and productivity of clinical development, using a refreshed Clinical Program Productivity Index, while also providing insights on contributing factors and enablers.
The small molecule share of clinical trial starts has been steadily declining over the past decade, with the most significant drop observed in Phase III trials, where small molecule share fell from 65% in 2015 to 53% in 2024.
News here
Cassava Sciences must face malicious prosecution lawsuit over Alzheimer's drug
The biotechnology company Cassava Science failed on Wednesday to end a malicious prosecution lawsuit by doctors and short-sellers who expressed doubts about its experimental, soon-to-be-discontinued Alzheimer's drug Simufilam.
U.S. District Judge Jennifer Rochon in Manhattan said Adrian Heilbut, Jesse Brodkin, Enea Milioris, David Bredt and Geoffrey Pitt could try to prove that Cassava knew or should have known that it could not win its defamation lawsuit against them, which it dismissed in August.
News here
Intellectual Property
T 2275/22 (Glaucoma II/SANTEN) 25-02-2025
EP 3205334, derived from a divisional application published as EP 2772 249 A1, was granted based on sixteen claims. The patent was issued to Santen Pharmaceutical Co., Ltd. Richard Cooke opposed it. This opposition appears to have been filed by a straw man.
Documents cited as prior art
D1: EP1321144 A
D13: Timoptic ® Data Sheet, 2005
D16: Guideline on the categorisation of extension applications (EA) versus variations applications (V), October 2003, European Commission
The claim that was contested was as follows:
Claim 1 of auxiliary request 4 defined:
"An ophthalmic aqueous solution consisting of 0.0015% w/v tafluprost as an active ingredient, 0.075% w/v polysorbate 80, 0.05% w/v disodium edetate, 2.25% w/v glycerol, 0.2% w/v sodium dihydrogen phosphate dihydrate, water and pH adjusters as excipients in a unit dose container consisting essentially of low density polyethylene, wherein the pH adjuster is sodium hydroxide and/or hydrochloric acid, wherein 0.3 mL of the ophthalmic aqueous solution is filled in the body part of the unit dose container and inner volume of the unit dose container is 1 mL, and wherein the ophthalmic aqueous solution has a pH of from 5.0 to 6.7."
Claim 1 of auxiliary request 5 included all the features of claim 1 of auxiliary request 4 and additionally defined the following feature:
"the container being packaged into paper-coated aluminium-polyethylene foil."
Closest prior art
The document D1 was regarded as the closest prior art.
Document D1 examines the use of nonionic surfactants to enhance the solubility of prostaglandin derivatives and to inhibit their absorption by containers made from resinous materials. In this context, document D1 introduces a stability test for an example of an ophthalmic solution that includes 0.005% tafluprost, 0.05% polysorbate 80, and 0.05% EDTA salt (see Table 7), packaged in a container made of polypropylene.
It was undisputed that the composition of Table 7 in document D1 represented the closest prior art and that the subject matter of claim 1 of the main request differed from that composition in
- the lower amount of tafluprost, namely 0.0015% w/v instead of 0.005%
- the higher amount of polysorbate, namely 0.075% w/v instead of 0.05%
- the presence of 2.25% w/v of glycerol
- the presence of 0.2% w/v of sodium dihydrogen phosphate dihydrate
- the presence of the pH adjusters sodium hydroxide and/or hydrochloric acid,
- the pH of 5.0-6.7
- the unit dose container having an inner volume of 1 mL and filled with 0.3 mL of the solution
- the container consisting essentially of low density polyethylene (LDPE) instead of polypropylene
It was also common ground that, insofar as the definition of 0.05% w/v disodium edetate in claim 1 of the main request represented a difference from the 0.05% w/w disodium EDTA disclosed in document D1, this difference was of no relevance in assessing inventive step.
Objective Technical Problem
Considering the nature of the distinguishing features of the claimed subject matter and in light of the experimental results on file, the Board formulates the objective technical problem as the provision of a convenient formulation for the administration of tafluprost that is free of preservatives and combines reduced absorption of tafluprost by the container with adequate bioavailability for an effective low dose of tafluprost.
Assessment of the solution
As evidenced by document D16 and exemplified by document D13, unit dose formulations, including those in LDPE containers holding 0.2 mL of solution and wrapped in a foil laminate, are well known in the field of ophthalmic solutions.
Such unit dose formulations represent as a matter of course convenient administration forms which may dispense with preservatives typically present in formulations intended for multiple administration.
Faced with the objective technical problem identified the skilled person would therefore as a matter of obviousness consider the possibility of adapting the composition of Table 7 in document D1 to a unit dose formulation in a LDPE container as defined in claim 1 of the main request.
Document D1 explicitly teaches that nonionic surfactants, preferably polysorbate 80, inhibit the absorption of prostaglandin derivatives, such as tafluprost, by containers made from resinous materials, preferably polypropylene and polyethylene of either high or low density.
In this context, document D1 presents the results of a stability test indicating that after six months of storage at 40°C, the remaining concentration of tafluprost is 72% in a polyethylene container and 83% in a polypropylene container. Considering the effective absorption inhibition by polysorbate 80 described in document D1, the skilled person would not be discouraged from adapting the composition of Table 7 in document D1 to a unit dose formulation, as defined in claim 1 of the main request, despite the less favorable surface-to-volume ratio of such unit dose formulations.
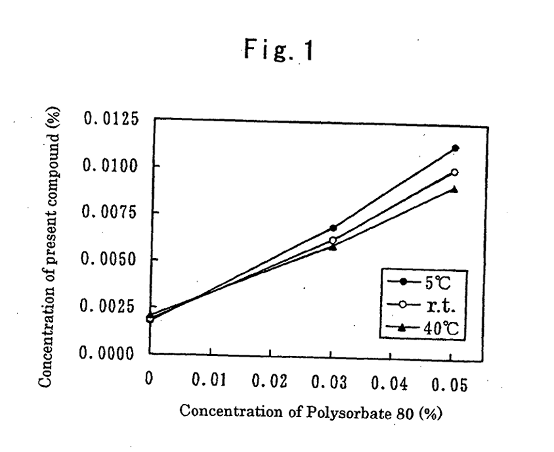
The patent proprietors argued that according to Figure 1 of document D1 tafluprost is already soluble up to a concentration of 0.02% without the presence of a nonionic surfactant.
According to the patentee, the skilled person would not expect any additional effect from increasing the concentration of polysorbate 80 when the concentration of tafluprost is lowered to the level of 0.0015, as defined in claim 1 of the main request.
However, Figure 1 of document D1 concerns the effect of the nonionic surfactant on the solubility of the prostaglandin derivative and not the effect on the absorption of the tafluprost by the container.
The effect of the nonionic surfactant on the absorption of the prostaglandin derivative is instead demonstrated in document D1 in Table 2 in relation to a composition comprising 0.001% tafluprost.
Therefore, considering the known effect of polysorbate 80 as stated in document D1, only routine experimentation is necessary to confirm the reduction of tafluprost absorption due to the increased polysorbate 80 concentration of 0.075%, as specified in the claim of the remaining request.
Consequently, the argument of the patent proprietors based on the solubility data from Figure 1 of document D1 is deemed unconvincing, leading to the revocation of the patent.
According to news articles from 2009, Merck paid an undisclosed fee along with milestones and royalty payments based on future sales of tafluprost (both preserved and preservative-free formulations) in exchange for exclusive commercial rights to tafluprost in Western Europe (excluding Germany), North America, South America, and Africa. Santen will retain commercial rights to tafluprost in most countries in Eastern Europe, Northern Europe, and the Asia Pacific, including Japan. Merck will provide promotional support to Santen in Germany.
Tafluprost, marketed as Taflotan, received marketing authorization in Europe for the treatment of glaucoma on June 15, 2016. The product is currently in a phase of marketing exclusivity until June 2026. An approved PIP (Paediatric Investigation Plan / Paediatric exclusivity) extends this period by six months. Consequently, the revocation of this patent is likely to create early launch opportunities of two to two and a half years for generic companies.