Competitive Generic Therapy (CGT) approvals and AbbVie Biotherapeutics Inc. vs Assistant Controller Of Patents (Delhi HC decision)
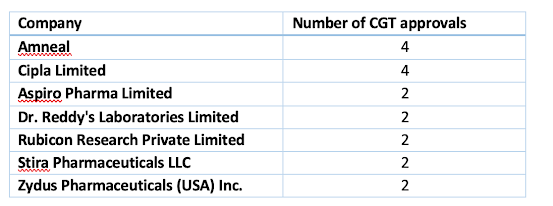
Contents Competitive Generic Therapy (CGT) approvals General information CytoSorbents Files Appeal with U.S. FDA for Supervisory Review of its De Novo Request for DrugSorb™-ATR Enlarged Board of Appeal harmonises EPO and UPC legislation 6 Intellectual Property AbbVie Biotherapeutics Inc. & Anr. vs Assistant Controller Of Patents- (Telisotuzumab vedotin) 7 Competitive Generic Therapy (CGT) approvals We follow CGT approvals. CGT designation: The Office of Generic Drugs provides a public list of all approved abbreviated new drug applications (ANDAs) for drug products with a Competitive Generic Therapy (CGT) designation and indicates which ANDAs on this list cover drug products eligible for CGT exclusivity under section 505(j)(5)(B)(v) of the FD&C Act, of 180 days. This list is updated bi-weekly with new ANDA approvals where at least one of the drug products, such as one of the strengths in the approved application, received a CGT designation. Eligible for CGT Exc...


