Competitive Generic Therapy (CGT) approvals and AbbVie Biotherapeutics Inc. vs Assistant Controller Of Patents (Delhi HC decision)
Contents
Competitive Generic Therapy (CGT) approvals
General information
CytoSorbents Files Appeal with U.S. FDA for Supervisory Review of its De Novo Request for DrugSorb™-ATR
Enlarged Board of Appeal harmonises EPO and UPC legislation 6
Intellectual Property
AbbVie Biotherapeutics Inc. & Anr. vs Assistant Controller Of Patents- (Telisotuzumab vedotin) 7
Competitive Generic Therapy (CGT) approvals
We follow CGT approvals.
CGT designation:
The Office of Generic Drugs provides a public list of all approved abbreviated new drug applications (ANDAs) for drug products with a Competitive Generic Therapy (CGT) designation and indicates which ANDAs on this list cover drug products eligible for CGT exclusivity under section 505(j)(5)(B)(v) of the FD&C Act, of 180 days. This list is updated bi-weekly with new ANDA approvals where at least one of the drug products, such as one of the strengths in the approved application, received a CGT designation.
Eligible for CGT Exclusivity
Certain products in ANDAs designated as CGTs can qualify for CGT exclusivity if the applicant is a “first approved applicant” as defined in section 505(j)(5)(B)(v) of the FD&C Act. There is only one exclusivity period available for each CGT, and receiving a CGT designation does not guarantee eligibility for CGT exclusivity. For example, a drug product in an ANDA that receives a CGT designation will not be eligible for CGT exclusivity if there are unexpired patents or exclusivities listed in the Orange Book at the time of the original submission of the ANDA for the CGT-designated drug product.
CGT Exclusivity Forfeiture
A drug product could qualify for CGT exclusivity upon approval, but it might lose that eligibility if not commercially marketed within 75 days of the ANDA approval date.
CGT approval details:
The top companies that sought the most CGT approvals in the six months are
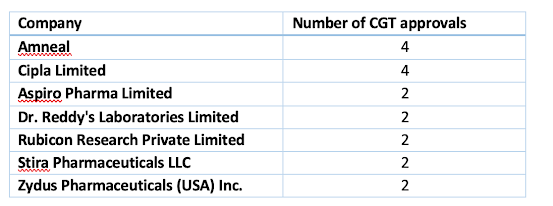
CGT approvals in the last 6 months.




General information
CytoSorbents Files Appeal with U.S. FDA for Supervisory Review of its De Novo Request for DrugSorb™-ATR
CytoSorbents Corporation, a leader in the treatment of life-threatening conditions in the intensive care unit and cardiac surgery using blood purification, today announced that on June 18, 2025, it filed a request for supervisory review (administrative appeal) with the U.S. Food and Drug Administration (FDA) under 21 CFR 10.75, of the De Novo Denial Letter issued on April 25, 2025 for CytoSorbents' DrugSorb-ATR Device, (the denial letter). DrugSorb-ATR, which previously received Breakthrough Device Designation from the FDA, is designed to reduce the severity of bleeding in patients undergoing coronary artery bypass grafting (CABG) surgery within two days of stopping the antiplatelet drug Brilinta® (ticagrelor, AstraZeneca), a commonly used blood thinner.
News here
Enlarged Board of Appeal harmonises EPO and UPC legislation.
When assessing the patentability of an invention, the description and drawings of an application must be considered to interpret the claims. With this recent decision, the Enlarged Board of Appeal has also brought the EPO's case law in line with the UPC.
News here
Intellectual Property
AbbVie Biotherapeutics Inc. & Anr. vs Assistant Controller Of Patents- (Telisotuzumab vedotin)
Case Overview:
The case before the Delhi High Court involves an appeal against an order by the Assistant Controller of Patents and Designs, which denied a patent application filed by Abbvie Biotherapeutics Inc. The patent application in question, numbered 201817047767, is titled "ANTI-cMet ANTIBODY DRUG CONJUGATES AND METHODS FOR THEIR USE." The appeal is filed under Section 117A of the Patents Act, 1970.
This patent application pertains to Telisotuzumab vedotin, marketed as Emrelis. In India, the injections, 20 mg or 100 mg are sold as a lyophilised powder in a single-dose vial. It is manufactured and marketed by AbbVie Inc.
Background Facts:
AbbVie Biotherapeutics Inc. and AbbVie Inc. are biopharmaceutical companies. Their patent application was filed as a national phase application in India, originating from a Patent Cooperation Treaty (PCT) application (PCT/US2017/033176). The application claims priority from a US patent application (US 62/337,796). The First Examination Report (FER) raised objections, including a lack of industrial applicability under Section 2(1)(j) and non-patentability under Section 3(i) of the Patents Act. A hearing was held, and written submissions were filed, but the patent application was ultimately refused under sections 2(1)(j), 3(i), and 59(1) of the Act. A subsequent review petition was dismissed as time-barred.
Claims:
Original Claim in PCT
A method of treating a solid tumor cancer that overexpresses cMet, comprising administering to a human subject having said cancer an anti-cMet antibody drug conjugate ("ADC") in an amount and for a period of time sufficient to provide a therapeutic benefit.
Amended claim
Amended claim filed with reply to FER (First Examination Report) on 05/05/2021, amended by submitting Form-13.
• An anti-cMet antibody drug conjugate (“ADC”), wherein the drug conjugate is monomethyl auristatin E (“MMAE”), and the ADC has the following structure:
wherein Ab is an anti-cMet antibody comprising a VH chain comprising three CDRs, namely VH CDR #1 (SEQ ID NO:112), VH CDR #2 (SEQ ID NO:113) and VH CDR #3 (SEQ ID NO: 114); a VL chain comprising three CDRs, namely VL CDR #1 (SEQ ID NO:115), VL CDR #2 (SEQ ID NO:116) and VL CDR #3 (SEQ ID NO:117); and a modified hinge region of SEQ ID NO:170, n has a value ranging from 2 to 8, and attachment to the Ab is via a thioether linkage formed with a sulfhydryl group of a cysteine residue.
Appellant's Submissions:
The appellant (AbbVie) argues that they did not lose the opportunity to amend the claims simply because amendments weren't made at the PCT stage. They cite Sections 57(6) and 59 of the Act, which allow amendments during the patent application process. They contend that since the amended claims directed to anti-cMet drug conjugates are disclosed in the patent specification, these should be permissible under Section 59(1), and the objections under Sections 2(1)(j) and 3(i) should be waived. They rely on a previous court decision (Allergan Inc. v. Controller of Patents) with similar facts.
Respondent's Submissions:
The respondent (Assistant Controller of Patents) argues that under Section 138(4) of the Act, a PCT national phase application should be identical to what was filed in the PCT, with limited exceptions. Since the appellants didn't amend claims at the PCT level, they entered the national phase with the same claims. The respondent argues that the revised claims were still method-of-treatment claims, and none were product claims. Further, the proposed amendments were beyond the scope of amendment under Section 59(1). The respondent relies on a previous court judgment (Nippon A & L Inc. v. Controller of Patents) and asserts that the Controller's order was well-reasoned.
Court's Analysis and Findings:
- The court reviews the claims filed as part of the PCT application, noting that the 137 claims included seven independent claims related to methods of treatment. The court emphasises that the specification focused heavily on therapeutic methods using anti-cMet antibody-drug conjugates (ADCs), including dosing schedules, dosage ranges, and strategies for resistant tumors. It finds that the original invention was intended and claimed as a method of treatment, with no standalone product/composition claim. The court finds that the original claims define "A method of treating a human subject having a NSCLC tumor with an IHC score of at least 2+ or an H score of 150 or greater…"
- The court states the amendments were rejected by the Controller as being impermissible under Section 59(1) of the Act. According to the Controller, the amendments sought by the applicant are not in the nature of correction or explanation or disclaimer and result in an enlargement of the scope of the claims than the originally filed claims.
- The court explains that claims can only be amended if they are not in nature of incorporating an actual fact, but it's a clever move to avoid the provisions of section 59 (1) of the Act which expressly bars the amendment which results in enlargement of scope of the claims than the originally filed claims.
- The court finds that the controller had concerns when the "Product without any limitation of method of treatment..." has larger scope than the "Product limited by its use in a specific method of treatment".
Key Legal Principles:
- Section 59 of the Patents Act: This section governs amendments to patent applications, specifying that amendments can only be made by way of disclaimer, correction, or explanation, and cannot broaden the scope of the claims.
- Section 3(i) of the Patents Act: This section excludes methods of treatment of humans from patentability.
- Section 2(1)(j) of the Patents Act: This section defines "invention" and implies requirements for industrial applicability.
- Section 138(4) of the Patents Act: This section governs PCT National Phase Applications.
- Coordinate Bench Precedents: The court refers to previous decisions (Nippon and Ovid) to guide the interpretation of Section 59.
Court's Decision:
The court ultimately upholds the Controller's decision, finding no infirmity in the order. The court agrees that the proposed amendments broaden the scope of the original claims and are therefore not permissible under Section 59(1) of the Act. The court also finds that the unamended claims are in the nature of a method of treatment (excluded under Section 3(i)) and do not fulfill the requirements for industrial application (Section 2(1)(j)). As a result, the appeal is dismissed.
In essence, the court's decision rests on the principle that patent applicants cannot use amendments to fundamentally alter the nature of their invention or broaden the scope of their claims beyond what was originally disclosed and intended in the patent specification. The court agreed with the Controller that the proposed changes would give the applicant rights to things that were not originally intended by the language of the patent.
Decision here



