Recent ANDA approvals and HQ Specialty Pharma Corp. Vs Fresenius Kabi USA, L.L.C
Contents
Recent ANDA approvals
General information
US FDA Will Not Pull Teva And MSN Hetlioz ANDAs, Rejects Vanda Citizen Petitions
Recent FDA Drug Approval Process Events
Intellectual Property
HQ Specialty Pharma Corp. Vs Fresenius Kabi USA, L.L.C.
Recent ANDA approvals
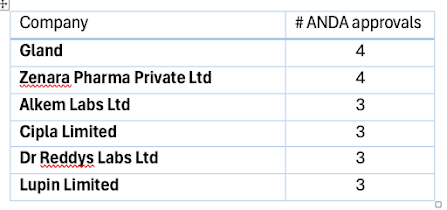
We follow ANDA approvals. In June 2025, there are approximately 103 ANDA approvals and 21 tentative approvals. The top companies that received approvals were as follows.Some of our other observations are as follows.
General information
US FDA Will Not Pull Teva And MSN Hetlioz ANDAs, Rejects Vanda Citizen Petitions
The FDA concluded that generic tasimelteon products held by Teva and MSN Labs meet all statutory bioequivalence requirements and there was no “mistake” or untrue statement in the original ANDA approvals. News hereRecent FDA Drug Approval Process Events
Stay informed on the latest biotech catalysts and regulatory activity with MarketBeat’s FDA Drug Approval Calendar. This resource tracks key milestones including Prescription Drug User Fee Act (PDUFA) dates, New Drug Applications (NDAs), Biologics License Applications (BLAs), Complete Response Letters (CRLs), and other FDA-related announcements. Whether you're monitoring upcoming approvals or reviewing recent FDA decisions, this calendar provides a clear view of the drug development landscape. News hereIntellectual Property
HQ Specialty Pharma Corp. Vs Fresenius Kabi USA, L.L.C.
Case Overview The case involves a patent dispute in the United States District Court for the District of Delaware between HQ Specialty Pharma Corp. and WG Critical Care, LLC (collectively, "Plaintiffs") and Fresenius Kabi USA, L.L.C. ("Defendant"). The dispute concerns U.S. Patent No. 10,130,646 (the "'646 patent"), which relates to a terminally sterilised calcium gluconate solution packaged in a free-flex plastic bag. The plaintiffs, HQ Speciality Pharma, alleged that Fresenius' calcium gluconate bag product infringed claims 1, 2, and 3 of the '646 patent. Fresenius counterclaimed, asserting the patent was invalid and unenforceable. Fresenius stipulated that it would infringe the patent if the claims were valid and enforceable. Background Facts This case concerns inventorship. Accurate inventorship under US patent law is essential because incorrect inventor listings can render a patent invalid or unenforceable. Inventorship determines ownership, and only the inventor(s) or their assignees are eligible to file a patent application. A patent may be invalidated if an inventor is omitted or if someone not involved in the invention's creation is listed as an inventor. HQ listed the patents below in the Orange Book for calcium gluconate The '646 patent is owned by HQ Specialty Pharma Corp. and names Joseph Pizza as the sole inventor. The patented invention is a ready-to-use bag for hospitals to administer intravenous calcium gluconate treatment to patients. The plaintiffs, i.e., HQ Specialty Pharma, filed the action on December 3, 2021. The approval date of HQ for 1GM/50ML (20MG/ML) product was in October 2018, but the `646 patent was listed in OB in October 2021.The FDA approved Fresenius Kabi's Calcium Gluconate in Sodium Chloride Injection on June 17, 2021. This approval was for their sNDA 208418/S-007, which added new presentations in Freeflex bags. Before Fresenius Kabi’s (FK) approval, HQ (i.e., the plaintiff) had not listed the patent in OB, and hence, there was no necessity for patent certification by FK. This is not classical P-IV litigation under the HW Act.
From August 26 to 30, 2024, a five-day jury trial took place, during which the plaintiffs (HQ) sought to prove damages, while Fresenius attempted to invalidate the asserted claims on grounds of obviousness and improper inventorship.
Claims:
1. A terminally sterilised aqueous calcium gluconate solution comprising:
sodium chloride; and
1 to 15 wt. % calcium gluconate and from 1 to 19 wt. parts of calcium saccharate per 100 wt. parts of calcium gluconate packaged in a flexible plastic container with the remainder water,
wherein
the flexible plastic container is a bag, and
the solution has a pH of from 6 to 8.2.
2. The terminally sterilized aqueous calcium gluconate solution of claim 1, wherein the solution comprises 19.6 mg/ml of calcium gluconate monohydrate, about 0.9 mg/ml of calcium D-saccharate, and about 6.75 mg/ml sodium chloride.
3. The terminally sterilized aqueous calcium gluconate solution of claim 2, wherein the solution has a shelf of at least about 24 months when stored at 25° C.
The jury concluded that Fresenius failed to prove that the asserted claims were obvious and found claim 1 invalid due to improper inventorship. However, they also determined that dependent claims 2 and 3 were invalid because of improper inventorship. The jury did not award damages, as the parties agreed that damages would only be considered if all claims were valid. The court entered judgment based on the jury's verdict on September 16, 2024.
The plaintiffs (HQ) moved to correct inventorship on September 11, 2024, and both parties filed motions for judgment as a matter of law on October 15, 2024.
Pending Motions
The court is considering four post-trial motions:
1. Plaintiffs' (HQ Specialty ) motion for judgment as a matter of law.
2. Defendant's (FK) motion for judgment as a matter of law, or alternatively, for a new trial.
3. Plaintiffs' (HQ) motion to correct inventorship.
4. Plaintiffs' (HQ) motion to alter the judgment.
Legal Standards
- The court outlines the legal standards for judgment as a matter of law, a new trial, correcting inventorship, and altering or amending a judgment. A judgment as a matter of law can be granted if the court finds that a reasonable jury would not have had a legally sufficient evidentiary basis to find for the party on an issue. A motion for a new trial can be granted if the jury's verdict is against the clear weight of the evidence, if new evidence exists that would likely alter the outcome, if there was improper conduct by an attorney or the court, or if the jury's verdict was facially inconsistent.
- Correction of inventorship is permissible if an inventor has been erroneously omitted from a patent. The party seeking correction must show by clear and convincing evidence that a joint inventor should have been listed. A motion to alter or amend a judgment can be granted if there is an intervening change in controlling law, new evidence, or a need to correct clear error of law or prevent manifest injustice.
- Plaintiffs' Motion for Judgment as a Matter of Law: The plaintiffs HQ argue that Fresenius did not introduce sufficient evidence to sustain the jury's verdict of improper inventorship for claims 2 and 3 and that no reasonable jury could find that anyone other than Dr. Sergio Dusci contributed as an unnamed co-inventor to claims 2 and 3.
- The court finds that there was substantial evidence that Dr. Dusci made a significant contribution to the patented invention, specifically the 6.75 mg/ml sodium chloride claim limitation of claims 2 and 3. Mr. Pizza, the named inventor, testified that he did not develop the specific amount of calcium gluconate or the chemical concentrations. Dr. Dusci, under whose direction InfoRLife worked, insisted on an osmolality level to make a better drug for patients and rejected the industry standard sodium chloride mixture.
- However, the court grants the motion in part, finding that Fresenius failed to present substantial evidence that anyone other than Mr. Pizza and Dr. Dusci made inventor-worthy contributions to the '646 patent.
Fresenius' Motion for Judgment as a Matter of Law or New Trial: Fresenius contends that the '646 patent was obvious and that it deserves a new trial due to three errors during the trial.
- The court denies Fresenius' motion for judgment as a matter of law, finding that the jury's verdict of non-obviousness was supported by substantial evidence. The court notes Dr. Zhao testified there was no reasonable expectation of success considering the uncertainties and risks, and that prior art did not motivate a skilled artisan to combine references to form the claimed invention.
- The court also denies Fresenius' motion for a new trial, finding that the jury's verdict was not against the great weight of the evidence.
Plaintiffs' (HQ) Motion to Correct Inventorship: The plaintiffs move to correct inventorship to add Dr. Dusci as a named inventor on the patent.
- The court grants the plaintiffs' motion, finding that their declarations are sufficient for correction under the statute. The court rejects Fresenius' arguments that the omission of Dr. Dusci was intentional and that the affidavits contain conditional assertions.
Plaintiffs' (HQ) Motion to Alter the Judgment: The plaintiffs move to amend the judgment of invalidity, arguing that the '646 patent is not invalid for lack of improper inventorship, now that the inventorship has been corrected.
- The court grants the plaintiffs' (HQ) motion, finding that the '646 patent is no longer invalid. An amended judgment will issue following the memorandum opinion.
Conclusion
The court grants in part and denies in part the plaintiffs' (HQ) renewed motion for judgment as a matter of law. The court rejects the defendant's (FK) motion for judgment as a matter of law or a new trial. The court grants the plaintiffs' motion to correct inventorship and to alter the judgment.
Key Outcomes
- Dr. Sergio Dusci will be added as a co-inventor on the '646 patent.
- The '646 patent is no longer considered invalid.
- The case will proceed with an amended judgment that reflects the corrected inventorship and validity of the patent.